Norovirus
General Information
Norovirus is a very contagious virus that is the leading cause of vomiting and diarrhea from acute gastroenteritis. It is sometimes called the “stomach flu” or the “stomach bug.” However, norovirus illness is not related to the flu (the flu is caused by the influenza virus). Worldwide, norovirus is the cause of about 1 out of every 5 cases of acute gastroenteritis that lead to vomiting and diarrhea.
Transmission
Norovirus is transmitted primarily by the fecal-oral route by direct person to person contact and contact with contaminated food, water or surfaces. You are most contagious when you have symptoms but can continue to spread the virus for 2 weeks or more after symptoms have resolved. It is the leading cause of outbreaks in healthcare. Healthcare facilities and other institutional settings (e.g., daycare centers, schools, etc.) are particularly at-risk for outbreaks but outbreaks can also occur on cruise ships and within restaurants and schools. Outbreaks occur throughout the year but are most common from November to April.
Symptoms
Symptoms usually develop 12 to 48 hours after exposure. The symptoms of norovirus include:
- Nausea
- Vomiting
- Diarrhea, stomach cramps
- Fever
- Headache
- Body aches
- Dehydration
Most people with norovirus illness get better within 1 to 3 days. The illness is usually brief for those individuals who are otherwise healthy. Young children under 5, adults 65 and older, and others who suffer from medical illnesses are most at risk for severe or prolonged infection.
Diagnosis
Diagnostic tests for norovirus detect viral RNA (genetic material) or viral antigen. Tests are available at all public health laboratories and many clinical laboratories.
Most tests use reverse transcription real-time polymerase chain reaction (PCR) assays to detect norovirus. PCR assays can be used to test the following for norovirus:
- Stool
- Vomitus
- Food
- Water
- Environmental specimen
Rapid commercial enzyme immunoassays (EIAs) that detect norovirus antigen in stool samples are also available, but these tests have poor sensitivity (50% to 75%) and are generally not recommended for testing single samples from sporadic cases of gastroenteritis.
Infection Prevention in Healthcare
In a healthcare facility, patients and residents with suspected norovirus should be placed in a private room on Contact Precautions. Two patients/residents with the same disease can share a room (cohort). Other prevention measures include:
- Follow hand-hygiene guidelines. Carefully wash hands with soap and water during outbreaks, Alcohol based hand sanitizer can be used in addition to soap and water, but should not replace washing with soap and water if hands are soiled
- Use gowns and gloves when in contact with patients who are symptomatic
- Isolate patients/residents for a minimum of 48 hours after symptom resolution but consider longer periods of isolation for complex medical patients as they may have prolonged symptoms and viral shedding
- Healthcare workers displaying signs and symptoms of gastroenteritis should be excluded from work until a minimum of 48 hours after symptoms have resolved (Consult facility Occupational Health and Safety to determine when it is safe to return to work)
- Rapidly assess individuals with gastroenteritis and isolate (if applicable)
- During an outbreak, utilize an EPA registered disinfectant with a Norovirus claim
EPA List G. - In all settings (healthcare and non-healthcare settings), do not prepare or handle food, or care for others until at least 2 days (48 hours) after symptom resolution to reduce the risk of norovirus transmission.
Cleaning and Disinfection
Norovirus is a small non-enveloped (not easy to kill) virus. Perform cleaning and disinfection of frequently touched environmental surfaces and shared equipment using EPA-registered products with label claims for use in healthcare. Follow the manufacturer’s recommendations for application and contact times. Refer to the EPA list of products with activity against norovirus on their website. When choosing a disinfectant, consider safety profile and the need for personal protective equipment. For example, bleach is effective against norovirus but there are other effective and safer options.
During an outbreak, increase the frequency of cleaning and disinfection of patient care areas especially frequently touched surfaces to twice or three times daily. Avoid the use of upholstered furniture and carpets in care areas and ensure privacy curtains are changed when visibly soiled and at discharge. Clean and disinfect the entire area immediately if contaminated with vomit or diarrhea. Handle linens and waste carefully and do not agitate to avoid dispersal of the virus.
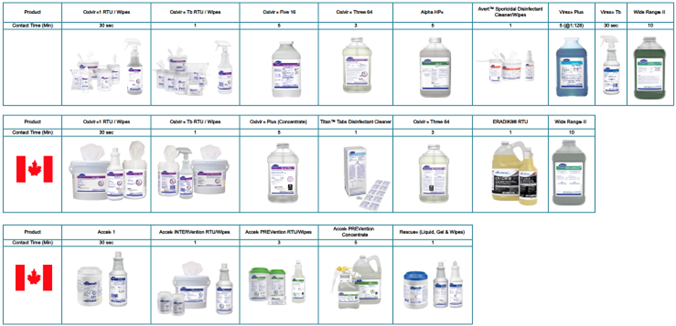
Norovirus is susceptible to the following Diversey disinfectants:
References:
CDC Guideline for the Prevention and Control of Norovirus Gastroenteritis Outbreaks in Healthcare Settings, February 15, 2017
https://www.cdc.gov/norovirus/data-research/index.html
https://www.cdc.gov/norovirus/causes/index.html
https://www.cdc.gov/norovirus/about/index.html
https://www.cdc.gov/infection-control/hcp/norovirus-guidelines/summary-recommendations.html
https://www.canada.ca/en/public-health/services/food-poisoning/norovirus/health-professionals.html
Reviewed and Revised: January 2025

